At the annual RSC Coordination and Organometallic Discussion Group Christmas meeting (UCD Dublin), everyone was asked to select an element as the focus of their talk, to celebrate the end of the International Year of the Periodic Table. Joe gave a talk celebrating the versatility of ruthenium complexes. The talk brought together two different project, linked by this transition metal.

Joe with his PhD supervisor Thorri and Dawn Barry 

IYPT presents for attendees
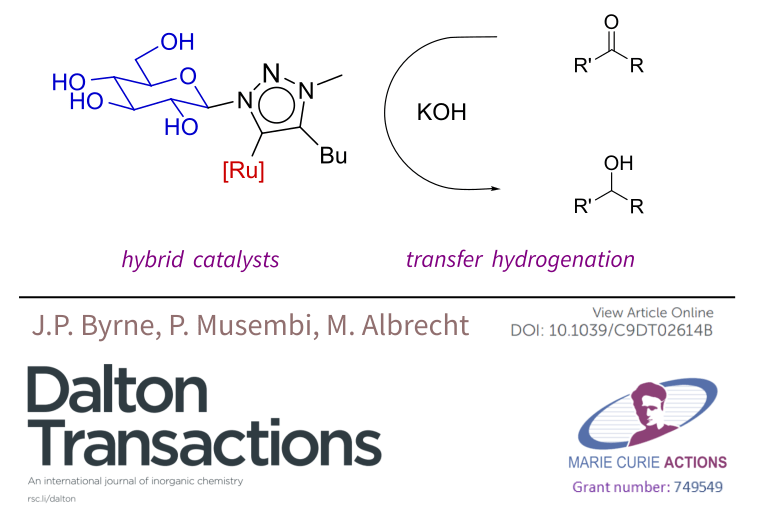
He spoke about the carbohydrate-functionalised Ru-NHC catalysts which were published recently in Dalton Transactions, as well as some Ru(II) coordination complexes (also featuring carbohydrates) which have shown antimicrobial activity (unpublished results, collaboration with Prof Thorri Gunnlaugsson and Dr Ciaran O’Reilly).
It was a very festive meeting, with inorganic chemists from all over Ireland and the UK in attendance – a great chance to catch up with old friends and new.