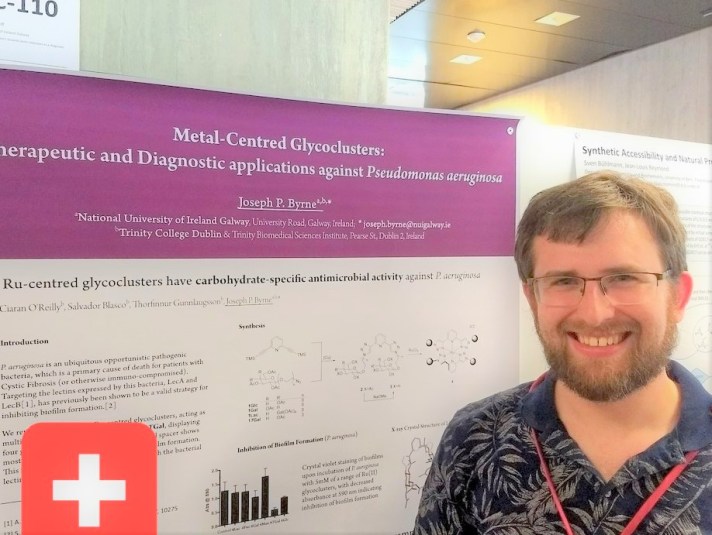
Our new article, published in RSC Advances (a Gold Open Access journal), describes a series of new ruthenium-centred glycoclusters, which present four carbohydrate motifs around a three-dimensional octahedral scaffold. Multivalent glycoclusters have previously shown the ability to inhibit the carbohydrate-binding proteins which are produced by bacterium P. aeruginosa. Gordon Cooke’s group in TU Dublin tested these new compounds for their ability to inhibit growth of biofilm by P. aeruginosa and we observed that complex 8Gal, with flexible arms between the scaffold core and the galactose motif gave up to 80% inhibition of biofilm, when compared to the control – the other complexes and the ligand did not show antimicrobial activity. We propose that this activity is due to the ability of galactose to interact with the carbohydrate-binding protein LecA.

We thank Science Foundation Ireland for financial support for this work, as well as UCD School of Medicine’s SSRA Scheme, where preliminary studies began.